- Alkanes, the simplest and most fundamental class of hydrocarbons, are characterized by their single covalent bonds between carbon atoms.
- This section delves into the methods of preparation, key chemical reactions, and the diverse applications of alkanes.
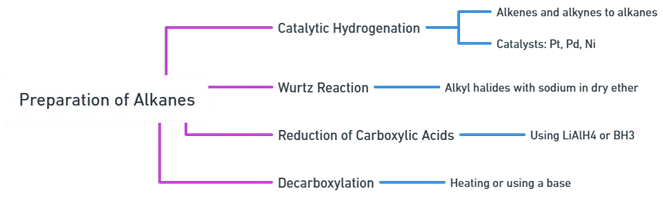
General Methods of Preparation
Advertisements
-
Catalytic Hydrogenation:
- Alkenes and alkynes are converted to alkanes by the addition of hydrogen in the presence of a catalyst such as platinum (Pt), palladium (Pd), or nickel (Ni).
- Example: Ethene (CH2=CH2) is hydrogenated to ethane (CH3-CH3) using Pt, Pd, or Ni as a catalyst.
-
Wurtz Reaction:
- This involves the reaction of alkyl halides with sodium metal in dry ether to produce alkanes.
- Example: Ethyl bromide (CH3CH2Br) reacts with sodium (Na) to form butane (CH3CH2-CH2CH3) and sodium bromide (NaBr).
-
Reduction of Carboxylic Acids:
- Carboxylic acids are reduced to alkanes using strong reducing agents like lithium aluminum hydride (LiAlH4) or borane (BH3).
- Example: Ethanoic acid (CH3COOH) is reduced to ethane (CH3-CH3) using LiAlH4.
-
Decarboxylation:
- Carboxylic acids are decarboxylated to alkanes by heating or using a base, releasing carbon dioxide (CO2).
- Example: Ethanoic acid (CH3COOH) undergoes decarboxylation to produce methane (CH4) and CO2.
Chemical Reactions of Alkanes
- Alkanes are saturated hydrocarbons, meaning they consist of carbon and hydrogen atoms bonded together by single covalent bonds.
- Due to their relatively inert nature, alkanes exhibit limited reactivity compared to other classes of organic compounds.
- However, they can undergo a few important reactions:
Advertisements
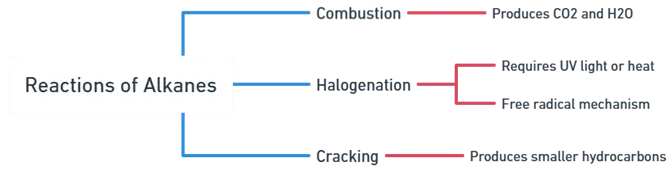
1. Combustion
-
- Alkanes readily undergo combustion reactions in the presence of oxygen to produce carbon dioxide and water.
- The general equation for the complete combustion of an alkane, such as methane (CH₄), is as follows:
- Alkane + Oxygen → Carbon Dioxide + Water
- For example, the combustion of methane is:
- CH₄ + 2O₂ → CO₂ + 2H₂O
Advertisements
2. Halogenation
-
- Alkanes can undergo halogenation, where halogens (such as chlorine or bromine) replace hydrogen atoms in the alkane molecule. This reaction requires ultraviolet (UV) light or heat to initiate.
- The reaction proceeds through a free radical mechanism.
- For example, the halogenation of methane with chlorine gas (Cl₂) proceeds as follows:
- CH₄ + Cl₂ (UV light/heat) → CH₃Cl + HCl
- This reaction can continue, leading to the formation of products such as:
- Dichloromethane (CH₂Cl₂)
- Trichloromethane (chloroform, CHCl₃)
- Tetrachloromethane (carbon tetrachloride, CCl₄)
3. Cracking
-
- Alkanes can undergo thermal decomposition, known as cracking, to produce smaller hydrocarbons.
- This process is often used in the petroleum industry to obtain shorter-chain hydrocarbons with higher commercial value.
- For example, the cracking of octane (C₈H₁₈) may produce ethene (C₂H₄) and propene (C₃H₆):
- C₈H₁₈ → C₂H₄ + C₃H₆ + other products
Applications of Alkanes
- Alkanes have widespread applications across various industries due to their availability and chemical properties:
- Fuels: Key components of natural gas, gasoline, diesel, and kerosene, essential for heating, transportation, and power generation.
- Petrochemical Industry: Used as raw materials for producing plastics, solvents, and lubricants, vital in manufacturing and industrial processes.
- Pharmaceuticals: Serve as solvents or starting materials in the synthesis of drugs.
- Cosmetics: Used as solvents, emollients, and thickeners in lotions, creams, and makeup.
- Food Industry: Utilized as carriers for flavors and in food-grade waxes to protect and preserve produce.
Advertisements

