-
Definition of Parenteral Products
- Parenteral Products refer to pharmaceutical preparations intended for administration by routes that bypass the digestive system, primarily through injections.
- This includes:
- Intravenous (IV): Directly into a vein.
- Intramuscular (IM): Into a muscle.
- Subcutaneous (SC): Under the skin.
- Intradermal (ID): Into the dermis layer of the skin.
- Other Routes: Such as epidural, intrathecal, and intra-articular.
- Parenteral administration is chosen for its rapid onset of action, precise dosage control, and suitability for drugs that are poorly absorbed orally or are inactivated by the gastrointestinal environment.
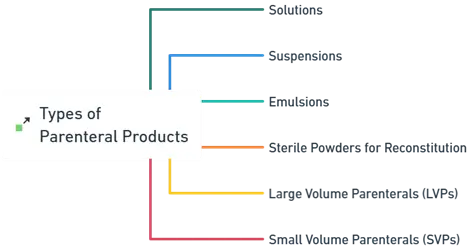
Types of Parenteral Products:
-
Solutions
- Clear, homogeneous liquid preparations.
- E.g., normal saline, dextrose solution, antibiotic solutions.
-
Suspensions
- Contain active pharmaceutical ingredients (API) suspended in a suitable vehicle.
- E.g., steroid hormone suspensions, certain vaccines.
- Must remain uniform upon shaking and must not settle too rapidly.
-
Emulsions
- Oil-in-water (O/W) or water-in-oil (W/O) dispersions.
- Used for certain drugs requiring lipophilic environments, e.g., some parenteral nutrition formulations.
-
Sterile Powders for Reconstitution
- Lyophilized or freeze-dried powders that must be reconstituted with a suitable diluent (e.g., WFI—Water for Injection) before use.
- Often used for drugs unstable in solution.
-
Large Volume Parenterals (LVPs)
- Typically, volumes >100 mL (e.g., 250 mL, 500 mL, 1000 mL).
- Used as IV infusions for fluid and electrolyte replenishment, total parenteral nutrition, etc.
-
Small Volume Parenterals (SVPs)
- Typically, volumes ≤100 mL.
- Include ampoules, vials, prefilled syringes.
Advantages:
- Rapid onset of action: Bypasses GI tract, enabling drugs to reach systemic circulation quickly.
- Complete bioavailability: Particularly IV route (100
- Controlled therapy: Suitable for patients unable to take oral medications (e.g., unconscious, GI disorders).
- Localized effect: Certain routes (IM, SC) can be used for sustained release at a local site.
Limitations:
- Sterility requirements: Must be free from microbial contamination.
- High production cost: Requires specialized aseptic facilities and equipment.
- Invasive: Associated with pain, fear, and risk of infection (e.g., at injection site).
- Trained personnel: Required for administration (especially for IV injections).
- Limited stability: Some drugs are unstable in solution; require special packaging or reconstitution.

